I see many petrochemical refiners struggle with impurities. I understand their frustration. If we fix these problems, we can transform the entire refining process.
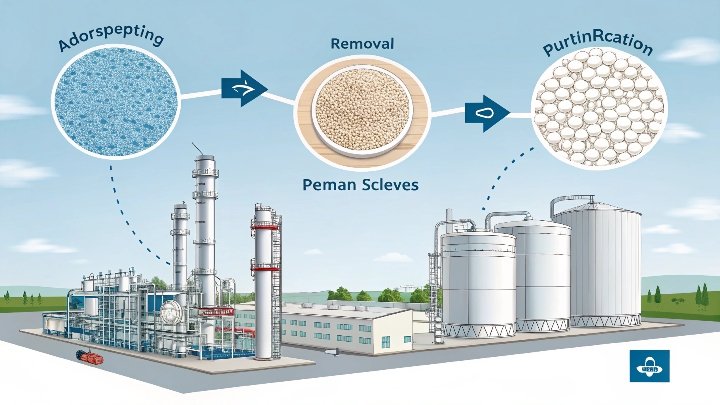
Molecular sieves can solve refinement challenges by selectively trapping unwanted molecules. These tiny adsorbents use precise pore sizes to remove impurities from feedstocks. They create cleaner fuels and plastics, boosting efficiency and sustainability. With lower energy use and reduced waste, they promise a bright future in petrochemical processes.
I remember when I first learned about molecular sieves. I was amazed at how something so small could have such a big impact. I realized that the future of refining would not just be about removing more impurities. It would also be about optimizing every step of the process. I like to compare them to a super-smart filter that helps maximize product quality. In this article, I will explore how molecular sieves work, why they are crucial, and how we can push their boundaries in the petrochemical industry. Join me on this journey into the tiny world of molecular sieves.
Molecular Sieves: The Tiny Titans of Petrochemical Purification?
I see these sieves as the unsung heroes of refining. They quietly trap contaminants, so products can be cleaner and processes more reliable.
Molecular sieves feature uniform pores that selectively adsorb molecules based on size or polarity. This targeted approach helps remove water, sulfur, and other troublesome substances, ensuring high-purity output.
I want to unpack how molecular sieves achieve their remarkable adsorption powers. First, these sieves have crystalline structures with pores of specific diameters. These pores work like tiny gates that let certain molecules enter while blocking others. This selective property is crucial when we aim to remove undesired impurities in petrochemical streams. For example, water molecules often cause corrosion and process inefficiencies, but molecular sieves can capture them before they wreak havoc. They can also target other contaminants like sulfur compounds, which can cause product discoloration or emission issues in fuels.
The Science Behind Adsorption
Molecular sieves rely on physical adsorption and electrostatic forces. Their frameworks are often made from materials like zeolite, which has an aluminosilicate structure. The arrangement of silicon, aluminum, and oxygen atoms creates a unique network of channels. Molecules enter these channels if they fit the pore size or if they are attracted by certain charge distributions. Once inside, they become trapped, which allows the main feedstock to proceed without interference.
Common Purification Challenges
In petrochemical processes, different feedstocks have different impurity profiles. Some streams contain moisture, others have heavy metals, and still others have organic sulfur or nitrogen. One molecular sieve might excel at absorbing moisture but may not handle sulfur efficiently. That is why we tailor the pore size and structure to specific challenges.
| Sieve Type | Target Impurity | Typical Application |
|---|---|---|
| 3A | Water | Drying of liquids |
| 4A | H2S | Sulfur removal |
| 13X | CO2 | Gas purification |
Selecting the right sieve is essential. It ensures consistent product quality, smoother operations, and fewer equipment failures. This clarity of design allows us to push petrochemical processes forward without sacrificing efficiency or safety.
From Crude to Clean: How Molecular Sieves Transform Petrochemicals?
I always believed that refining was about detail. Molecular sieves bring that detail, letting us remove contaminants at the source and set new cleanliness standards.
They streamline the conversion from crude to high-purity products. By adsorbing trace impurities, molecular sieves prevent side reactions, reduce byproduct formation, and help us meet strict regulations.
I want to show how molecular sieves shape each step in the journey from raw feedstock to final product. First, they remove water and other polar contaminants early in the refining process. This prevents corrosion in pipelines and storage tanks. It also reduces the risk of catalyst deactivation in subsequent treatment steps. Less downtime and maintenance mean smoother production flows, which leads to economic advantages. Next, molecular sieves help ensure that petrochemical intermediates meet strict quality requirements. For instance, if we are creating plastics, any excess moisture can impact polymerization. That can lower product consistency or cause lumps. By controlling these conditions, molecular sieves allow polymer processes to run more efficiently.
Why Impurity Removal Matters
Impurities can compromise the chemical reactions at the heart of petrochemical processes. When catalysts come into contact with substances like sulfur or basic nitrogen compounds, they can lose their activity. This spells costly regeneration or replacement. In some cases, these impurities cause safety hazards, such as flammable gas mixtures or toxic byproducts. By integrating molecular sieves in the flow, we can protect catalysts and reduce the risk of undesired events. This leads to fewer shutdowns and product recalls.
The Path to Cleaner Products
When I see how end products like plastics or fuels benefit from molecular sieve technology, I understand why they are so valuable. These small adsorbents protect feedstocks at every stage. They also reduce off-spec material that might otherwise become waste. And the big win is consistency. Customers need consistent performance from fuels, polymers, and specialty chemicals. Molecular sieves help keep that standard. These benefits make me believe that adopting advanced molecular sieve systems is the key to achieving better productivity and environmental responsibility.
Supercharging Efficiency: The Future of Molecular Sieve Refining?
I am excited about how new sieve materials can cut energy costs. With smarter designs, we may lower operating temperatures and pressures without sacrificing performance.
I think we can push boundaries with next-generation molecular sieves. They might help us capture carbon and recycle valuable byproducts. This promises a cleaner and more efficient petrochemical sector.
I see a future where molecular sieves move beyond simple impurity removal. They may become vital for large-scale carbon capture systems in petrochemical plants. By selectively adsorbing carbon dioxide, advanced sieves could reduce greenhouse gas emissions at their source. This would help us align with increasingly strict environmental standards while finding ways to reuse CO2 in other processes. I believe it can open doors to circular economy models, where waste gases become feedstocks for new products. For example, captured CO2 might be converted into valuable chemicals or used for enhanced oil recovery with lower environmental impact.
Innovative Materials
Researchers are exploring materials like metal-organic frameworks (MOFs) and advanced zeolites with tuned pore sizes. These can target very specific molecules, improving separation performance. They can also reduce the energy needed to regenerate the sieves. Traditional methods often require heating or vacuum conditions, but new materials may work at milder temperatures or use alternative regeneration techniques. This means lower operating costs and less downtime.
Automation and Data
I also think digitalization will drive new sieve applications. Monitoring adsorbent performance in real time allows for predictive maintenance. When sensors detect saturation, the system can automatically regenerate or switch to a fresh bed. This helps operators avoid bottlenecks and maintain consistent output. Paired with machine learning, these insights could guide us to optimize sieve selection, pore size distribution, and feedstock flow rates on the fly. This synergy of advanced materials and smart operations will likely reshape petrochemical refining. We could see more flexible plants that adapt quickly to feedstock variations while maintaining top product quality.
Conclusion
I believe molecular sieves hold the key to cleaner, more efficient petrochemical operations, opening opportunities for sustainability and innovation in every corner of the industry.