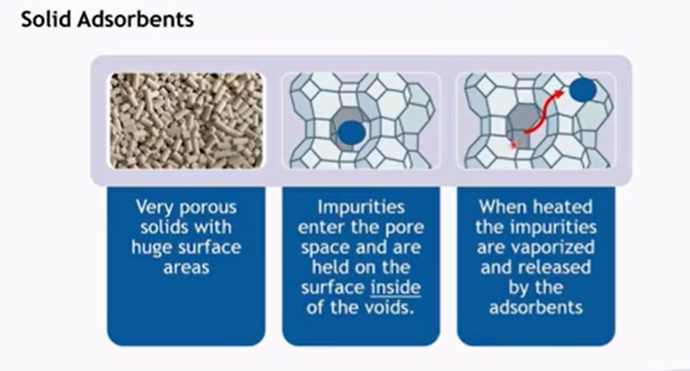
Molecular sieve dehydration is a process that relies on the use of molecular sieves, which are materials with tiny, uniform pores that can adsorb molecules based on their size and polarity. Here’s how the process works and potential issues that could arise:
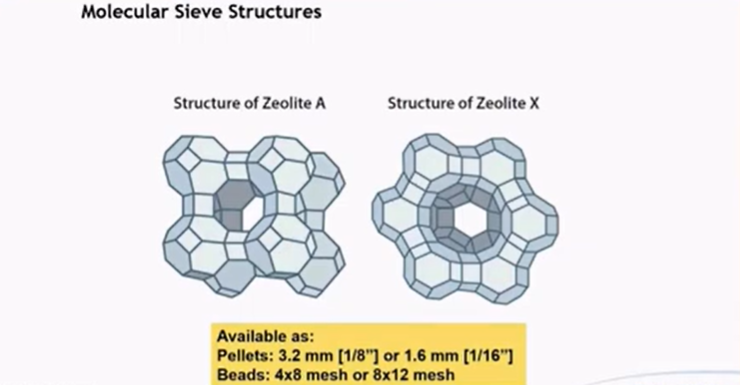
How Molecular Sieve Dehydration Works?
Adsorption:
Molecular sieves, typically made from aluminosilicates, have a highly porous structure with a uniform pore size (e.g., 3A, 4A, 5A).
When a liquid or gas containing water passes through the molecular sieve bed, water molecules, which are small and polar, are adsorbed into the pores of the molecular sieve.
This selective adsorption removes water from the stream, leaving the remaining molecules (which are too large to enter the pores) relatively unaffected.
Desorption (Regeneration):
Over time, the molecular sieve becomes saturated with water and loses its effectiveness.
To restore its adsorption capacity, the molecular sieve must be regenerated, usually by heating (thermal regeneration) or by reducing the pressure (pressure swing adsorption) to release the adsorbed water.
The desorbed water is then purged from the system, and the molecular sieve is ready for reuse.
What Could Go Wrong?
Saturation and Ineffective Dehydration:
If the molecular sieve is not properly regenerated or if the regeneration process is insufficient, the sieve will remain saturated with water.
This saturation leads to reduced dehydration efficiency, meaning the process will not remove water effectively, compromising the quality of the end product.
Chemical Degradation:
Some aggressive or high-temperature chemicals can degrade the molecular sieve, reducing its adsorption capacity or even causing it to break down.
Over time, exposure to certain chemicals might lead to irreversible damage, necessitating more frequent replacement of the sieve material.
Channeling and Maldistribution:
In fixed bed systems, improper packing or settling of the molecular sieve can cause channeling, where the fluid bypasses much of the bed.
Channeling reduces contact time between the fluid and the molecular sieve, leading to inadequate dehydration.
Contamination:
Contaminants such as oils, fine particulates, or other impurities can clog the pores of the molecular sieve, blocking access to the water molecules.
This contamination reduces the effective surface area of the sieve and thus its capacity to adsorb water.
Thermal Stress During Regeneration:
If the temperature used during the regeneration process is too high, it can damage the molecular sieve structure, causing cracking or loss of adsorption capacity.
Conversely, too low a temperature may fail to fully regenerate the sieve, leaving it partially saturated.
Inconsistent Feed Composition:
Variations in the feed composition (e.g., sudden increases in water content) can overload the molecular sieve, leading to breakthrough where water is no longer effectively removed.
Mechanical Failure:
Over time, the physical structure of the molecular sieve may degrade due to mechanical stress, leading to dust formation or breakdown of the material, which can cause pressure drop issues and reduce the effectiveness of the dehydration process.
By understanding these potential issues, operators can better manage the molecular sieve dehydration process, ensuring optimal performance and extending the life of the molecular sieves.