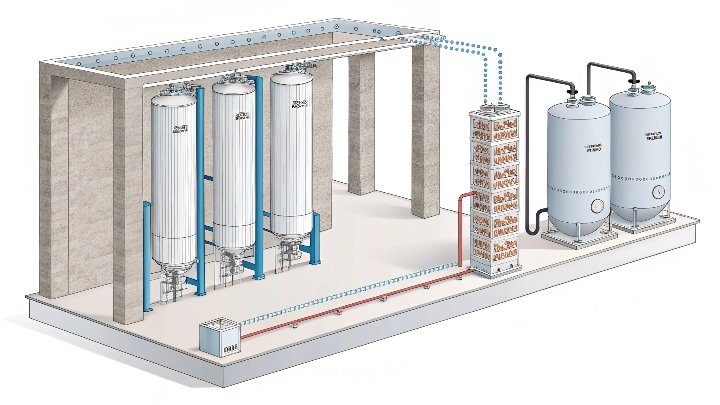
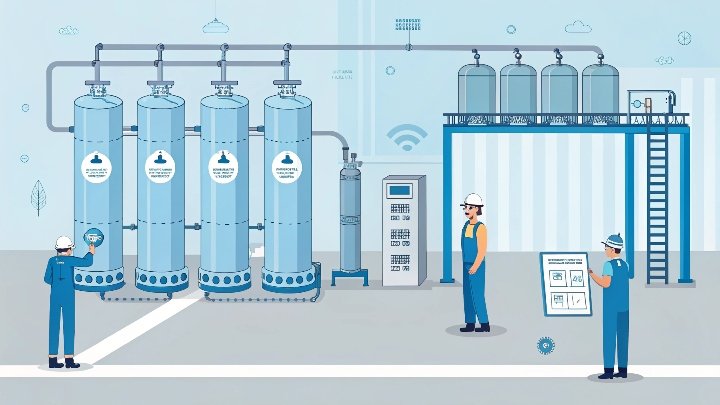
In the refining of butane, achieving high purity levels is crucial for various industrial applications. But how can molecular sieves contribute to this process?
Molecular sieves are essential in removing contaminants like water, carbon dioxide, and other hydrocarbons, ensuring butane meets purity standards. These adsorbents help create the high-quality butane required in different industries.
Refining butane requires several steps to meet the varying purity requirements of different applications, from 99.5% for commercial uses to even higher levels for more specific needs, like pharmaceuticals. Molecular sieves play a critical role in removing contaminants during this process, ensuring the butane is of the highest quality. In this article, we will explore how molecular sieves are used in the separation and purification of butane, focusing on their impact on purity, efficiency, and cost-effectiveness.
The Role of Molecular Sieves in Butane Separation: Enhancing Purity and Efficiency?
Purity is essential when refining butane, especially in industries like pharmaceuticals or aerosols. How do molecular sieves help enhance this purity?
Molecular sieves effectively remove impurities such as water, carbon dioxide, and other minor contaminants, allowing the production of ultra-high-purity butane needed for specialized applications.
In butane separation, the refining process must remove contaminants to achieve the required purity. For butane, impurities like water, carbon dioxide, and other trace hydrocarbons must be separated to meet stringent standards. Molecular sieves, specifically 5A and 13X types, are ideal for this purpose. These sieves are chosen because their uniform pore sizes enable them to selectively adsorb water and carbon dioxide without affecting the butane itself.
Through adsorption, molecular sieves allow for the separation of contaminants while maintaining the integrity of the butane. This ensures that the final product can be used in critical applications that require high purity. For instance, activated carbon, though effective in removing odors or hydrocarbons, cannot achieve the same level of selectivity and stability needed for such high-purity demands. In contrast, molecular sieves are superior due to their precise pore structure and higher thermal stability.
How Molecular Sieves Enhance Purity and Efficiency in Butane Refining
Molecular sieves are designed to adsorb specific molecules based on their size. Their unique structure allows them to selectively adsorb water and carbon dioxide while leaving butane untouched. This selectivity makes them the preferred choice over other adsorbents, such as activated carbon, which are less efficient and less stable in such demanding conditions. Below is a simple comparison:
| Adsorbent Type | Adsorption Capacity | Selectivity for Water and CO2 | Thermal Stability | Use in Butane Purification |
|---|---|---|---|---|
| Molecular Sieves | High | Very High | High | Essential for high-purity separation |
| Activated Carbon | Moderate | Low | Moderate | Suitable for odor control, not for ultra-purity |
The efficiency of molecular sieves helps improve the overall refining process by ensuring that butane can be processed more quickly and with fewer resources. This reduces the energy consumption and cost associated with the separation process, making it both environmentally friendly and economically viable.
Choosing the Right Molecular Sieve for Optimal Butane Purification?
How do you select the best molecular sieve for butane purification? This choice can significantly affect the efficiency and cost-effectiveness of the process.
The right molecular sieve is crucial for ensuring high-purity butane. 5A and 13X sieves are most commonly used due to their specific properties that allow effective removal of impurities like water and CO2.
When selecting a molecular sieve for butane purification, the choice depends on several factors, including the size of the pores and the specific impurities you need to target. The most commonly used molecular sieves in this process are 5A and 13X types. These sieves are especially effective at removing water and carbon dioxide from the butane stream.
Molecular sieves with a pore size of around 5 angstroms (5A) are excellent for adsorbing water, while 13X sieves, which have slightly larger pores, are better suited for removing both water and CO2. Understanding the composition of your crude butane and the purity requirements of your final product is critical when choosing the right molecular sieve. Using the wrong type of sieve can result in inefficiencies and higher operational costs.
Key Considerations for Choosing the Right Molecular Sieve
When selecting a molecular sieve for butane purification, keep the following factors in mind:
- Pore Size: Molecular sieves come in different pore sizes, each designed to target specific contaminants. For water and CO2 removal, 5A and 13X are most commonly used.
- Thermal Stability: Molecular sieves need to withstand high temperatures during the refining process without degrading or losing efficiency.
- Adsorption Capacity: The sieve must have enough adsorption capacity to handle the volume of contaminants in the butane without needing frequent replacement.
| Factor | 5A Molecular Sieve | 13X Molecular Sieve |
|---|---|---|
| Pore Size | 5 Å | 10 Å |
| Best for | Water Adsorption | CO2 & Water Adsorption |
| Thermal Stability | High | High |
| Typical Use | Butane Purification | Butane and CO2 Removal |
Choosing the right molecular sieve can not only ensure high-purity butane but also optimize the overall process, making it more cost-effective.
Molecular Sieve Technology: Reducing Energy Consumption and Costs?
How does molecular sieve technology contribute to reducing energy consumption and costs in butane refining?
By optimizing the purification process, molecular sieves reduce energy usage and operational costs, making them a highly efficient choice for industrial butane separation.
One of the primary benefits of using molecular sieves in butane purification is the reduction in energy consumption. Cryogenic distillation, which is often used for separating butane from other hydrocarbons, requires significant amounts of energy, particularly when operating at extremely low temperatures. Integrating molecular sieves into the process allows for more efficient separation, reducing the need for excessive energy input.
Moreover, the selective adsorption capabilities of molecular sieves make the overall refining process faster, as they can more quickly target and remove contaminants. This helps to reduce both the time and the energy required to achieve the desired purity. With fewer contaminants, the butane can be processed more efficiently, allowing for less downtime and lower energy costs.
The Efficiency and Cost Benefits of Molecular Sieves in Butane Refining
Molecular sieves reduce the need for energy-intensive processes, helping to lower operational costs. Their ability to selectively adsorb impurities ensures that only the highest quality butane is produced. This improves not only the purity of the final product but also the overall productivity of the refining process.
| Benefit | Effect of Molecular Sieves |
|---|---|
| Reduction in Energy Consumption | Significant |
| Increased Process Efficiency | High |
| Reduced Operational Costs | Noticeable |
By incorporating molecular sieves into the butane refining process, companies can achieve a more efficient and cost-effective operation, helping them stay competitive in a demanding industry.
Conclusion
Molecular sieves are vital in the purification of butane, enhancing its purity, efficiency, and cost-effectiveness. Their ability to remove water, CO2, and other impurities ensures the production of high-quality butane for various industrial applications.