You’ve probably seen those tiny "Do Not Eat" packets in shoe boxes or vitamin bottles. But did you know they’re secretly high-tech tools powering rockets and saving your morning coffee?
Molecular sieves are synthetic materials with microscopic pores that selectively trap molecules based on size and polarity. They’re used everywhere from drying electronics to filtering oxygen in spacecraft, making modern life possible through precise molecular control.
These unassuming crystals work like microscopic bouncers, deciding which molecules get in and which stay out. Let’s break down how they achieve this molecular magic – and why your coffee stays fresh longer because of technology developed for space stations.
What Exactly Are Molecular Sieves? The Tiny ‘Nanocages’ Powering Modern Industry
Imagine a sponge that only soaks up specific chemicals. Now shrink it to atomic scale – that’s your molecular sieve.
Molecular sieves are crystalline aluminosilicates with uniform nano-sized pores. Their cage-like structure (3-10 Å) acts as a filter, trapping molecules smaller than pore openings while excluding larger ones – like a subway turnstile for atoms.

The Atomic Architecture Behind Adsorption
These synthetic zeolites aren’t random sponges – they’re precision-engineered at the molecular level. Here’s what makes them unique:
| Feature | Traditional Desiccants | Molecular Sieves |
|---|---|---|
| Pore Uniformity | Random sizes | Exact ±0.1 Å |
| Adsorption Strength | Weak physical bonds | Targeted trapping |
| Moisture Capacity | 20-30% weight | Up to 22% weight |
| Regeneration Cycles | 5-10 times | 100+ cycles |
The magic lies in their repeating SiO₄ and AlO₄ tetrahedra. When aluminum replaces silicon, it creates negative charges balanced by cations (Na⁺, K⁺, Ca²⁺). These cations act like doorkeepers, determining which molecules can enter through size exclusion and electrostatic attraction.
How Do Molecular Sieves Work? The Science of Size-Selective ‘Molecular Traffic Control’
It’s not magic – it’s molecular judo. These crystals use physics to outsmart chemicals.
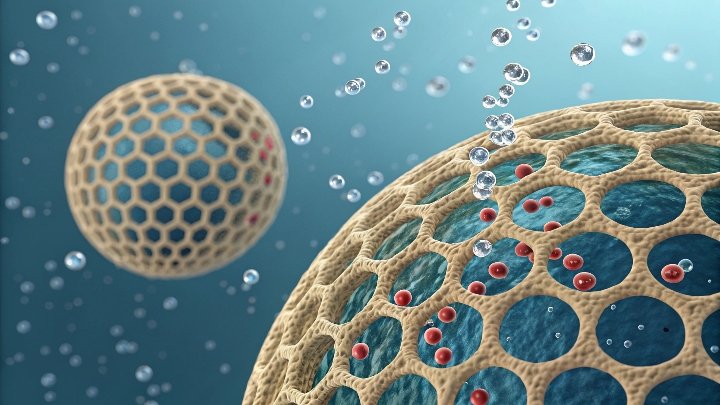
Molecular sieves adsorb molecules through size exclusion and surface attraction. Their uniform pores (3Å-10Å) act like sorting gates, trapping smaller molecules while letting larger ones pass – like separating marbles through different-sized sieves.

The Three-Step Molecular Tango
-
Size Filtering
Only molecules smaller than pore openings enter. Water (2.6Å) fits into 3A pores, ethanol (4.4Å) needs 4A sieves. -
Surface Capture
Polar molecules get stuck through electrostatic interactions. The AlO₄⁻ sites grab polar molecules like water (H₂O) or CO₂. -
Capacity Management
Each "cage" holds molecules until heat (250-350°C) or vacuum releases them. A 1kg 3A sieve can store 220g water – 5x more than silica gel.
From Coffee to Cosmos: 5 Unexpected Places Molecular Sieves Keep Our World Dry
Your morning routine is a molecular sieve showcase – from toothpaste cap to car dashboard.
Beyond industrial uses, molecular sieves protect medications, enable fuel cells, preserve museum artifacts, dehydrate astronaut suits, and even capture whiskey flavor compounds during aging.
The Invisible Protectors
-
Space Glove Liners
NASA uses 13X sieves (10Å pores) to absorb CO₂ and moisture in EVA suits. Each glove contains 50g of sieves – enough for 8-hour spacewalks. -
Whiskey Maturation
Distilleries use 3A sieves to remove fusel oils (large molecules causing harshness) without ethanol loss. Result: smoother bourbon in half the aging time. -
Vaccine Storage
Lyophilized mRNA vaccines require <1% humidity. 3A sieves in vial stoppers maintain dryness for 18+ months at room temperature. -
Electric Car Batteries
Li-ion batteries use molecular sieves to trap HF gas from electrolyte decomposition. Extends battery life by 40%. -
3D Printing Filament
Nylon filaments absorb water like sponges. 4A sieves in storage bags keep moisture <50ppm – preventing print failures.
Conclusion
Molecular sieves prove that big solutions come in small packages. From keeping your shoes dry to enabling Mars missions, these atomic-scale filters quietly shape our world – one molecule at a time.


