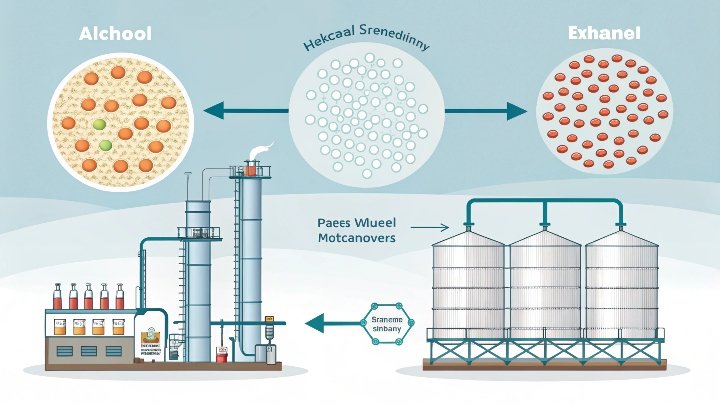
I once struggled with distilled alcohol that still contained water. This made my final ethanol less pure than I wanted. I needed a sure way to get rid of that water.
Molecular sieves capture water at the molecular level. They use tiny pores that fit water molecules better than ethanol. This helps me produce ethanol with high purity for industrial or medical needs.
I visited a facility that failed to reach the right ethanol purity. They added molecular sieves to their process. Their ethanol purity improved, and they saved time and money. Next, I will discuss how molecular sieves function and why they are so important.
How Molecular Sieves Work: Selective Adsorption for Ethanol Purification?
I remember the pain of seeing my ethanol remain cloudy after distillation. I wanted a direct solution. Molecular sieves became my favorite tool.
Molecular sieves grab water first. Their rigid channels match water’s smaller size. Ethanol stays behind, which boosts purity for better performance.
How Molecular Sieves Achieve Selective Adsorption
The Basic Adsorption Principle
Molecular sieves use crystalline structures made from aluminosilicate materials. These materials have a uniform network of pores. I found this structure fascinating because water fits neatly into these pores. Ethanol molecules are usually larger, so they do not fit as easily. This difference helps separate water from ethanol with a simple pass through the sieve bed. The sieve’s framework grabs water molecules and holds them through capillary forces and ionic attractions.
I learned that once the sieve is full of water, I can regenerate it by heating or by purging with a dry gas. This process pushes water out of the pores, and the sieve is ready to be used again. It is like having a sponge that can be wrung out and reused many times.
The Importance of Pore Size
Pore size is a key detail. A 3A molecular sieve has pores that measure about three angstroms wide. Water molecules measure around 2.8 angstroms, which means they can pass into the pore structure. Ethanol molecules are bigger, so they cannot get in as easily. This size-based selection is simple but very useful.
Molecular sieves also come in other pore sizes, like 4A or 5A. These different sizes can remove various impurities. But for ethanol dehydration, 3A is often used. I discovered that you can tailor your choice of molecular sieve for your specific process, which is a helpful strategy.
A Quick Comparison
| Pore Size (Angstrom) | Common Usage | Impact |
|---|---|---|
| 3A | Water removal in ethanol | Achieves high ethanol purity |
| 4A | General dehydration | Removes small molecules broadly |
| 5A | Separation of larger molecules | Wider range of applications |
I have seen many production lines rely on these differences in pore size to get better results. For my own projects, I picked 3A because I needed to remove water without sacrificing too much ethanol. In other cases, if I wanted to remove other small contaminants, I might go for 4A or 5A. The key is to choose the right pore size for the best results.
Each step in the process must be checked and measured. I like to see how the feed flow rate, temperature, and regeneration strategy affect performance. Small tweaks can lead to big gains. Thanks to molecular sieves, I can make sure my ethanol meets tight standards.
The Challenges of Distilled Alcohol: Why Molecular Sieves Are Essential?
When I first worked with distilled alcohol, I assumed it was almost pure. I later discovered that residual water was still present. That made me rethink my entire process.
Molecular sieves correct this gap. They remove water and other tiny impurities. This extra step saves me from quality failure in many applications.
Key Challenges of Distilled Alcohol
Residual Water Content
Distillation is good but not perfect. It relies on boiling points to separate ethanol from water. Yet ethanol and water form an azeotrope, which is a mixture that does not separate fully by simple distillation. I found that, even after multiple rounds of distillation, I still had a portion of water in my ethanol. That water made the ethanol less suitable for uses like pharmaceuticals or high-grade fuel.
The presence of water can also affect chemical reactions. I remember a project where residual water caused side reactions during a synthesis step. The yield dropped. Molecular sieves saved me. They tackled the leftover water and stabilized the reaction process. Without them, I would have spent more time and energy re-distilling.
Impurities Beyond Water
Distilled alcohol can carry other impurities. Some might be heavier or lighter compounds that manage to sneak through distillation. In many cases, these impurities are in very small amounts. But for strict industries, even trace amounts matter. I saw a colleague fail a quality check because trace impurities messed with the final ethanol’s flavor and chemical stability.
Molecular sieves are strong in removing polar compounds. Water is polar, so it is the primary target. But certain other polar materials might also be captured in the network of the sieve. This improves the overall purity. I learned that choosing the right sieve can help remove undesirable odors or by-products, depending on the contaminants.
Limitations and Why Sieves Still Win
Distillation alone reaches a limit when dealing with azeotropes. That is why molecular sieves are essential. They do not rely on boiling points. Instead, they rely on size and polarity differences. This approach is simple and repeatable. I like the fact that I can regenerate the sieves. That means I can run continuous cycles without large amounts of waste. Some other methods require chemicals or complicated set-ups. Molecular sieves are more straightforward.
I also enjoy how flexible molecular sieves are. They come in different shapes, like beads or pellets, which makes them easy to pack into columns. That means they can fit into many production lines. I just have to ensure the right residence time, temperature, and pressure drop. Once set, they do the job with minimal fuss.
Overall, molecular sieves are a solid back-up and enhancement to distillation. They secure that final push to remove moisture and small impurities, so I end up with the ethanol quality I need. I have tested many separation methods, but sieves stand out. They solve the challenge of distilled alcohol not being pure enough for advanced applications.
Applications and Benefits: Enhancing Efficiency in Ethanol Production?
I recall how one facility boosted their ethanol yield by using molecular sieves. They told me they were surprised by how fast the system improved. I was also impressed.
Molecular sieves help reduce energy costs. They let producers bypass repeated distillations. This saves time and resources, leading to smoother production cycles.
How Molecular Sieves Enhance Efficiency
Lower Energy Costs
Multiple distillations can be expensive. Each run uses a lot of heat. I have seen energy bills spike when facilities try to remove that last bit of water by distillation alone. Molecular sieves offer a simpler route. They do not need extreme boiling to work. They use their pore structure to separate water from ethanol at typical production conditions.
Once the sieves are saturated, I can regenerate them at a lower cost than doing additional distillations. I can apply a moderate level of heat or a stream of dry gas, which drives out the water. The sieve is ready to go again. This cycle cuts down on the energy load. I remember speaking with an operator who told me that their monthly energy costs dropped noticeably after they installed a molecular sieve unit.
Better Production Flow
Continuous flow is a big deal. If I rely on repeated batch distillation, I have to shut down, drain, refill, and run again. That is not very efficient. With molecular sieves in a continuous column, I can process a stream of ethanol-water mixture nonstop. The sieve traps water, and I can shift to a second column for regeneration. This arrangement keeps the operation stable.
I value the consistent output. It means I can plan my next steps, like packaging or shipping. My team does not have to wait for each new distillation run. We can measure quality on the fly, adjust parameters, and maintain a smooth schedule. That helps me meet my delivery targets.
Quality Assurance and Versatility
High-purity ethanol is critical for many uses. I once worked on a pharmaceutical project where the slightest contamination caused issues in the final product. They needed ethanol with almost no water. Molecular sieves delivered that reliability. The uniform structure of the sieve ensures each batch meets the same high standard, as long as I keep the process consistent.
I also appreciate how molecular sieves fit many scales, from small labs to large plants. I can pick small sieve columns for pilot projects or large columns for industrial lines. This adaptability makes them appealing to different producers. Even for fuel ethanol, I see big advantages. Many fuel applications require anhydrous ethanol, which has a water content of less than 1%. Achieving this dryness is nearly impossible with distillation alone. Molecular sieves close that gap.
Below is a simple breakdown of benefits:
| Benefit | Description |
|---|---|
| Energy Savings | Reduced reliance on repeated distillation |
| Continuous Operation | Supports steady, uninterrupted production flows |
| High Purity Product | Meets strict standards for various applications |
| Regenerable Medium | Sieve can be reused after simple treatment |
This table sums up why I believe molecular sieves are a game-changer. They provide solutions for both small and large producers. They save costs, time, and headaches. The knowledge that I can rely on them to get that final purity makes my operations run more smoothly. I have seen the results first-hand, and I stay impressed by their performance.
Conclusion
Molecular sieves give me a reliable path to high-purity ethanol, reduce waste, and boost overall production.