I see many professionals stuck when they try to remove CO₂ from car exhaust. I want to solve that problem. Let me explain how.
Commonly, 13X molecular sieve is chosen for CO₂ removal because it has a high adsorption capacity. But real operating conditions also matter, so I suggest testing it.
I believe this question has many layers. I will walk you through each key point. I hope you find clarity and practical value in the following sections.
What are the molecular sieves used to remove CO2?
I notice some confusion about how to pick the right molecular sieve. Let me share my insights on these options.
Many CO₂ adsorbers rely on 13X or 5A. 13X usually adsorbs more CO₂, so I often recommend it for general applications.
I have been working with molecular sieves for a long time. I remember my early days in a factory that produced these tiny pellets. I was surprised by the differences between 5A, 13X, and other variants. I noticed how each sieve responded to temperature changes, gas concentrations, and operating pressures. That experience shaped my view on selecting the best material for each scenario.
I want to break down the two popular types that often come up for CO₂ capture. Type 5A has a smaller pore size, around 5 angstrom. It adsorbs many small molecules, but it might not hold as much CO₂ as 13X. On the other hand, 13X has a pore size around 10 angstrom. It often shows a higher capacity for CO₂ because it allows more molecules to enter and bind to its internal surfaces.
I have also seen people using specialty zeolites with custom pore structures. But those are less common in everyday use because of higher costs. When I speak to designers, they often ask about the best choice for removing CO₂ in high-volume conditions. I always say 13X is good if we run at normal temperatures. But if we have a high-temperature exhaust stream, we must consider potential performance drops. 13X can lose some efficiency at higher temperatures because CO₂ does not bind as strongly. That is why we might need to cool the exhaust gas or use a different strategy, such as a layered bed with different adsorbents.
When I plan an adsorption system, I look at a few key parameters: temperature, pressure, CO₂ concentration, and required removal efficiency. I have summarized these factors in the following table:
| Parameter | Effect on CO₂ Removal | Typical Approach |
|---|---|---|
| Temperature | Higher temperatures reduce adsorption capacity. | Use cooling or choose high-temperature adsorbents. |
| Pressure | Higher pressures often increase adsorption. | Use pressurized systems for better performance. |
| CO₂ Concentration | High concentrations may saturate adsorbent quickly. | Ensure enough adsorbent volume or regeneration cycles. |
| Required Purity | Tighter purity requirements demand more precise control. | Use advanced process designs or layered beds. |
I see some people assume that any molecular sieve can remove CO₂ effectively. But every product has its own optimal range. 5A may work for some smaller applications, while 13X excels in moderate temperature ranges and moderate CO₂ concentrations. In actual practice, I encourage extensive pilot testing. It is a good idea to confirm that the adsorbent will meet your exact operating needs.
I recall one project I handled where a client wanted to use 13X in a hot exhaust line without adequate cooling. The results were disappointing because the adsorption capacity plummeted with the hot inlet gas. We fixed it by installing a small heat exchanger that brought the temperature down to a more favorable range for 13X. That improved CO₂ capture significantly.
When you need to remove CO₂, consider each factor. Do not forget to weigh the cost of regeneration, too. 13X might demand more energy to regenerate because it can adsorb lots of CO₂, but also water and other components. That means the regeneration temperature might be higher, leading to more energy use.
In short, there are multiple molecular sieves for CO₂ removal, but 13X stands out if temperature is controlled. And it usually has the highest capacity among standard choices. This is why I like 13X when I design carbon capture processes at normal conditions.
What are the main components of automobile exhaust?
I see that many people only focus on CO₂. They forget about other exhaust components. Let me explain the basics of automotive emissions.
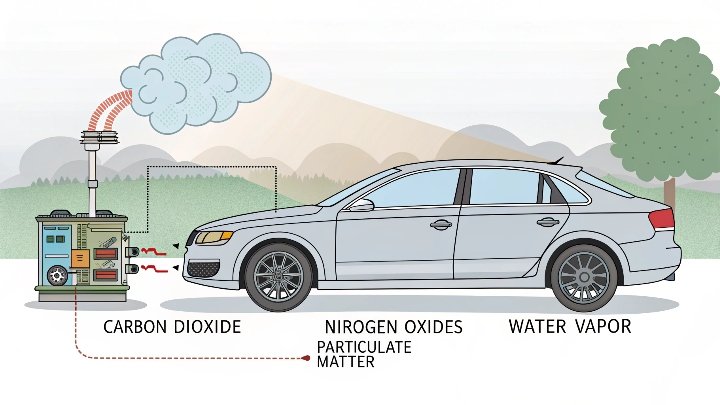
Automobile exhaust contains CO₂, water vapor, nitrogen, oxygen, carbon monoxide, hydrocarbons, and NOx. CO₂ is high in concentration and important to reduce.

I have spent a lot of time studying the composition of car exhaust. When I first measured exhaust gases in a lab, I was surprised at how many byproducts came out. Combustion inside an engine is complex. Fuel and air burn, and the result is a mixture of gases. CO₂ is often the main greenhouse gas. Water vapor is also common. But there are more toxic components like carbon monoxide (CO), unburned hydrocarbons (HCs), and oxides of nitrogen (NOx). All these matter because they contribute to pollution in different ways.
I learned that the levels of these pollutants vary based on the engine type and fuel quality. Gasoline engines tend to produce less soot, but they still have CO and NOx. Diesel engines create more soot, but they can sometimes have better fuel efficiency. This leads to less total CO₂ for the same distance traveled, though the exhaust can have other harmful particles.
CO₂ is a greenhouse gas that traps heat in the atmosphere. So many companies try to reduce CO₂ to curb climate change. But removing just CO₂ from the exhaust will not solve all air quality issues. That is why modern cars have catalytic converters that handle CO and hydrocarbons, turning them into less harmful substances. NOx control often needs special devices, such as selective catalytic reduction systems with urea injection or exhaust gas recirculation.
When I discuss molecular sieves for CO₂ capture, I must also acknowledge that you usually find these sieves in industrial settings. In typical car designs, it is impractical to place a giant canister of 13X in every vehicle. The operating temperature is high, and the volume of exhaust is large. So the molecular sieve would fill up quickly. Regeneration would be a challenge. That is why large-scale carbon capture for vehicles is still mostly a research and pilot concept. Commercial solutions might exist in niche applications or stationary engines where you can cycle or switch out adsorbent beds.
Still, knowing the main components of automobile exhaust helps me see the big picture. I can better appreciate why a single approach is not enough. We might trap CO₂ but still have to handle the other gases. If we pick 13X for CO₂, we must also confirm it does not get saturated too fast by water vapor or degrade if there are high sulfur compounds or other contaminants.
I have heard from some colleagues that they tried multi-bed systems where an initial bed captures moisture, then a 13X bed captures CO₂. This can work, but it adds complexity. The design must fit into the vehicle or the engine system. For stationary engines, it might be easier to set up. Either way, understanding the overall composition of exhaust helps me choose the right adsorbent strategy and plan the necessary pretreatment.
In short, car exhaust is not just CO₂. It is a mix of many substances. A targeted approach is necessary for each pollutant.
To capture carbon dioxide (CO₂) in automobile exhaust, selecting the right type of molecular sieve is the key.
I know that many people want to reduce CO₂ output. I believe the correct sieve can help, but we must think about conditions.
13X is more suitable for CO₂ capture at normal temperatures. But high-temperature exhaust may reduce its adsorption performance, so steps to manage that are important.

I have often recommended 13X to clients because it has a high CO₂ adsorption capacity and decent selectivity. However, no solution is perfect. Car exhaust streams are hot, often well above 100°C. This heat can lower the adsorption capacity of 13X. Also, if the CO₂ concentration is very high, the sieve can saturate quickly. Then you will have to regenerate it often. That is difficult to do continuously in a moving vehicle without an elaborate system.
I recall a test project I joined. The client wanted to attach an adsorbent canister to a bus exhaust to grab some CO₂. They hoped to reduce emissions in real-time. We tried 13X because it has strong CO₂ uptake at lower temperatures. But we found that at operating exhaust temperatures, the capacity plummeted. We had to incorporate a cooling unit. That improved adsorption, but it consumed energy. We had to weigh that energy cost against the emission benefits.
Then there is the matter of water vapor. Exhaust gas is often moist. 13X also adsorbs water, which can reduce its ability to adsorb CO₂. We needed a pre-drying step or a design that accounted for water. Another challenge was the large CO₂ fraction in the exhaust stream. If the exhaust has around 10-15% CO₂ (or more), then the sieve would fill quickly. We had to add more frequent regeneration steps.
Another factor is pressure. Many car exhaust systems operate near atmospheric pressure. Adsorption is more effective at higher pressure. Some designers have considered using a compressor to boost the pressure. But that adds weight and complexity. It might not be practical for everyday vehicles.
Despite these hurdles, I still think 13X is a good baseline choice if we can handle the temperature. In some stationary engine setups, 13X can be a valid option because there is space and infrastructure for cooling and regeneration. We can store the captured CO₂ for later processing or disposal. If you are dealing with a simple test system or a controlled environment, 13X gives you a good place to start. Testing is essential. I would not trust any single data sheet without real trials.
I have also encountered alternative adsorbents, like activated carbon or specialized zeolites. Activated carbon can handle some level of CO₂ capture, but often less selectively than 13X. Custom zeolites might have better temperature performance, but they come at a higher price. The best approach often depends on the priorities: Are we focusing on cost, capacity, selectivity, or temperature tolerance?
One more thing to keep in mind is regeneration. 13X can be regenerated by heating or purging with a lower-pressure gas or air. Doing that in a continuous vehicle operation scenario is tough. We might need multiple beds or a rotating system. This is simpler in large industrial processes, but not as easy for a personal car.
I believe future research might develop better adsorbents or incorporate integrative systems that capture and then chemically convert CO₂ on-board. But for now, 13X stands out as a robust starting point for CO₂ capture if we mitigate the high temperature. We must also factor in the water content and the large volume of exhaust gas. That is why I emphasize pilot tests and real-world data.
Conclusion
I see 13X as a strong choice for removing CO₂ from car exhaust, but we must handle the temperature and operating demands.